A downloadable study snapshot and updated ethics and regulatory documentation are now available for BeatPain Utah, an NIH Pragmatic Trials Collaboratory Trial.
A downloadable study snapshot and updated ethics and regulatory documentation are now available for BeatPain Utah, an NIH Pragmatic Trials Collaboratory Trial.
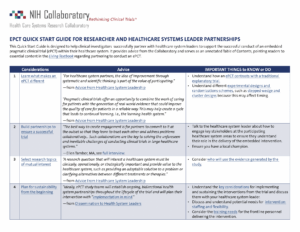
BeatPain Utah recently transitioned from the planning phase to the implementation phase. As part of the transition, the study team reviewed and updated the minutes of their initial ethics and regulatory consultation with the Ethics and Regulatory Core. The project is studying real-world implementation of a telehealth physical therapy strategy for patients with chronic back pain in primary care clinics of federally qualified health centers.
- Read the updated ethics and regulatory documentation, including information about the study team’s approach to informed consent and data monitoring and oversight.
- Also available is a new study snapshot for BeatPain Utah. This downloadable handout summarizes the study’s aims, lessons from the planning phase, and links to other resources from this innovative pragmatic clinical trial.
BeatPain Utah is supported by the NIH through the NIH Heal Initiative under an award from the National Institute of Nursing Research.




 NIH Collaboratory researchers in 2021 shared study results, generated new knowledge, and developed innovative research methods in pragmatic clinical trials. Their work included insights from the Coordinating Center and
NIH Collaboratory researchers in 2021 shared study results, generated new knowledge, and developed innovative research methods in pragmatic clinical trials. Their work included insights from the Coordinating Center and