 Ethics and regulatory onboarding documentation for 2 of the NIH Pragmatic Trials Collaboratory's newest trials is now available. The documents include meeting minutes and supplementary materials summarizing recent discussions of ethics and regulatory issues associated with the ARBOR-Telehealth and I CAN DO Surgical ACP studies.
Ethics and regulatory onboarding documentation for 2 of the NIH Pragmatic Trials Collaboratory's newest trials is now available. The documents include meeting minutes and supplementary materials summarizing recent discussions of ethics and regulatory issues associated with the ARBOR-Telehealth and I CAN DO Surgical ACP studies.
The consultations took place by video conference and included representation from the studies' principal investigators, members of the NIH Collaboratory's Ethics and Regulatory Core, NIH staff, and NIH Collaboratory Coordinating Center personnel. Both projects are in their planning phase.
ARBOR-Telehealth will evaluate the use of a telehealth physical therapy strategy for patients who present to primary care clinics with low back pain in rural communities. A secondary aim of the study is to compare the effectiveness of the study's risk-stratification approach.
- Learn more about ARBOR-Telehealth.
- View the ethics and regulatory documentation for ARBOR-Telehealth.
I CAN DO Surgical ACP will identify a system-based approach to help older adults undergoing elective surgery engage in advance care planning. The project will leverage the existing electronic health record and patient portal, PREPARE for Your Care materials to assist patients with completion of advance care planning, virtual healthcare navigators, and electronic nudges. Another goal of the study is to understand digital engagement, language, and social drivers of health that drive engagement in the intervention.
- Learn more about I CAN DO Surgical ACP.
- View the ethics and regulatory documentation for I CAN DO Surgical ACP.
Ethics and regulatory documentation for all of the NIH Collaboratory Trials is available on our Data and Resource Sharing page.



 In this Friday's PCT Grand Rounds, Roxana Mehran of the Icahn School of Medicine at Mount Sinai will present
In this Friday's PCT Grand Rounds, Roxana Mehran of the Icahn School of Medicine at Mount Sinai will present  Investigators from the NIH Pragmatic Trials Collaboratory in 2023 shared study results, generated new knowledge, and developed innovative methods in the design, conduct, and analysis of pragmatic clinical trials. Their work included insights from the Coordinating Center and
Investigators from the NIH Pragmatic Trials Collaboratory in 2023 shared study results, generated new knowledge, and developed innovative methods in the design, conduct, and analysis of pragmatic clinical trials. Their work included insights from the Coordinating Center and  In this Friday's PCT Grand Rounds, Ruth Engelberg, Erin Kross, and Robert Lee of the University of Washington will present
In this Friday's PCT Grand Rounds, Ruth Engelberg, Erin Kross, and Robert Lee of the University of Washington will present 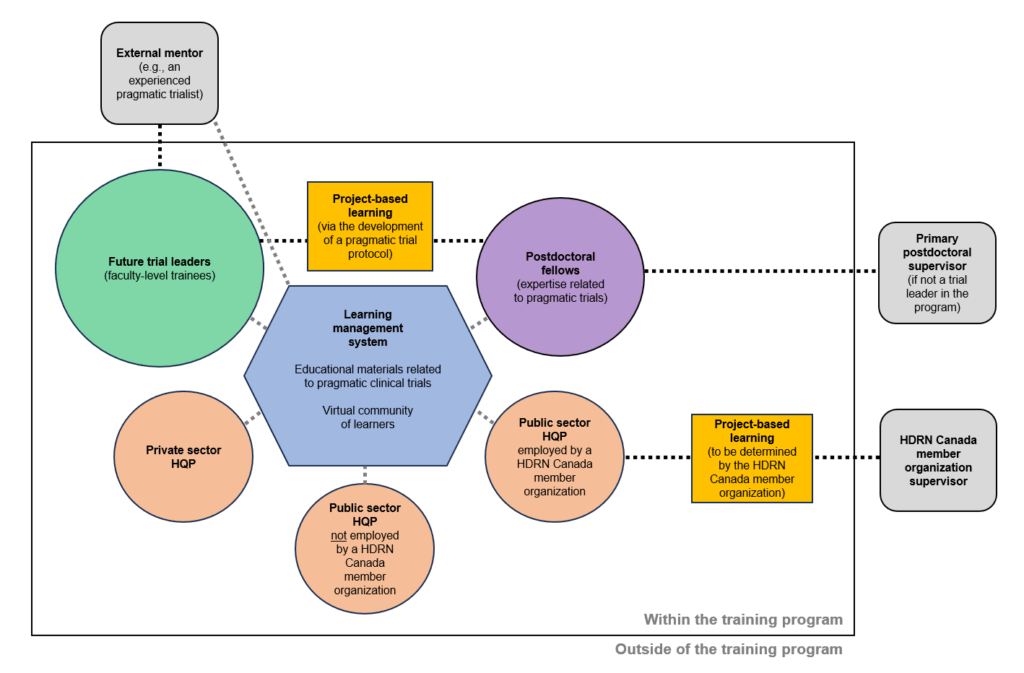 Health Data Research Network (HDRN) Canada is now accepting applications for its
Health Data Research Network (HDRN) Canada is now accepting applications for its  In a new episode of our Rethinking Clinical Trials podcast, Dr. Harlan Krumholz and members of his research team speak with host Dr. Lesley Curtis about lessons from the digital, decentralized, and democratized Yale PaxLC Trial. Krumholz and team presented on their experiences during the October 27 session of Grand Rounds.
In a new episode of our Rethinking Clinical Trials podcast, Dr. Harlan Krumholz and members of his research team speak with host Dr. Lesley Curtis about lessons from the digital, decentralized, and democratized Yale PaxLC Trial. Krumholz and team presented on their experiences during the October 27 session of Grand Rounds. The Health Care Systems Research Network (HCSRN) is accepting
The Health Care Systems Research Network (HCSRN) is accepting  The NIH Office of Disease Prevention will continue its Methods: Mind the Gap webinar series on Friday, December 8, with
The NIH Office of Disease Prevention will continue its Methods: Mind the Gap webinar series on Friday, December 8, with  In this Friday’s PCT Grand Rounds, Jim Hughes of the University of Washington will continue our special series, Advances in the Design and Analysis of Pragmatic Clinical Trials, with his presentation,
In this Friday’s PCT Grand Rounds, Jim Hughes of the University of Washington will continue our special series, Advances in the Design and Analysis of Pragmatic Clinical Trials, with his presentation,  The
The