At the NIH Collaboratory Steering Committee Meeting in May 2019, participants shared their perspectives on the evolving landscape of embedded pragmatic clinical trials (ePCTs). Three initiatives were presented: the Patient-Centered Outcomes Research Institute (PCORI), the NIH-DoD-VA Pain Management Collaboratory, and the HEAL (Helping to End Addiction Long-term) Initiative. Although many challenges remain, the conduct of ePCTs is gaining momentum, and the synergy between the initiatives, along with the fellowship they engender, will continue to help pave the way for more embedded pragmatic research in the future.
Dr. Ann Trontell, Associate Director of Clinical Effectiveness and Decision Science at PCORI, shared PCORI’s experience with pragmatic clinical studies. Since 2014, PCORI has awarded $494 million dollars for 43 pragmatic studies that range in size from 425 to 100,000 participants (median, approximately 1700). The studies include 2 observational, 27 individually randomized, and 14 cluster randomized trials in a wide range of therapeutic areas.
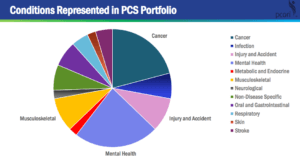
Dr. Trontell urged those developing proposals for pragmatic trials to make them fit for purpose, as opposed to emphasizing pragmatism, a theme echoed in the Developing a Compelling Grant Application chapter of the Living Textbook.
Dr. Robert Kerns, a director of the NIH-DoD-VA Pain Management Collaboratory, shared progress with pragmatic trials designed to evaluate whether evidence-based nonpharmacological approaches are effective for pain management among US military personnel and veterans.
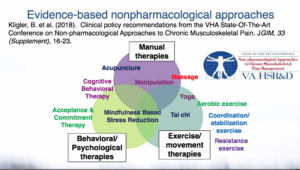
Modeled after the NIH Collaboratory, the Pain Management Collaboratory is supporting 11 projects through a 2-year planning phase and a 2- to 4-year implementation phase. Subject matter experts at the Pain Management Collaboratory Coordinating Center (PMC3) support the projects by sharing tools, best practices, and resources.
Dr. Wendy Weber, Program Officer for the NIH Collaboratory Coordinating Center, introduced the HEAL initiative, which is designed to enhance pain management and improve prevention and treatment strategies for opioid misuse and addiction. The goal of the initiative is to provide scientific solutions to the opioid crisis. It includes a set of large-scale pragmatic trials that will receive logistical and technical support from the NIH Collaboratory Coordinating Center.
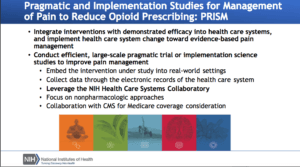
While experience with ePCTs is growing, many distinct challenges remain. As the conduct of ePCTs gains momentum, there is a rich opportunity to use collective experiences to refine best practices to real-world evidence generation and help solve urgent public health problems.
 The NIH this month published notice of a funding opportunity to support the next round of pragmatic clinical trials within the NIH-DoD-VA Pain Management Collaboratory (PMC).
The NIH this month published notice of a funding opportunity to support the next round of pragmatic clinical trials within the NIH-DoD-VA Pain Management Collaboratory (PMC).





