A recent impact report from the Tufts Center for the Study of Drug Development details how efforts to streamline clinical trial design appear to be yielding results [1]. Industry trial sponsors are using mechanisms such as facilitated review processes and adaptive trial designs to identify and address study feasibility issues, thereby reducing trial costs and administrative burden.
The center reviewed more than 20,000 procedures from recent phase 2 and 3 trials sponsored by 8 major pharmaceutical or biotechnology companies and conducted a survey with 83 industry executive respondents on experience with facilitated review processes.
Highlights from the analyses include the following:
- 21% of procedures in phase 2 trials and 31% in phase 3 trials were for “non-core” data that did not support primary or key secondary outcomes
- Non-core procedures accounted for, on average, one-quarter of a study budget
- >90% of non-core data collected were included in clinical study reports and regulatory submissions
- 21% of companies reported using simple adaptive trial designs to reduce costs and improve chances of study success; <10% reported using sophisticated adaptive designs
- 76% of surveyed companies reported using facilitated review processes within the past 5 years
- Many companies reported modest to major improvements in various measures of trial performance since implementing facilitated review processes (figure)
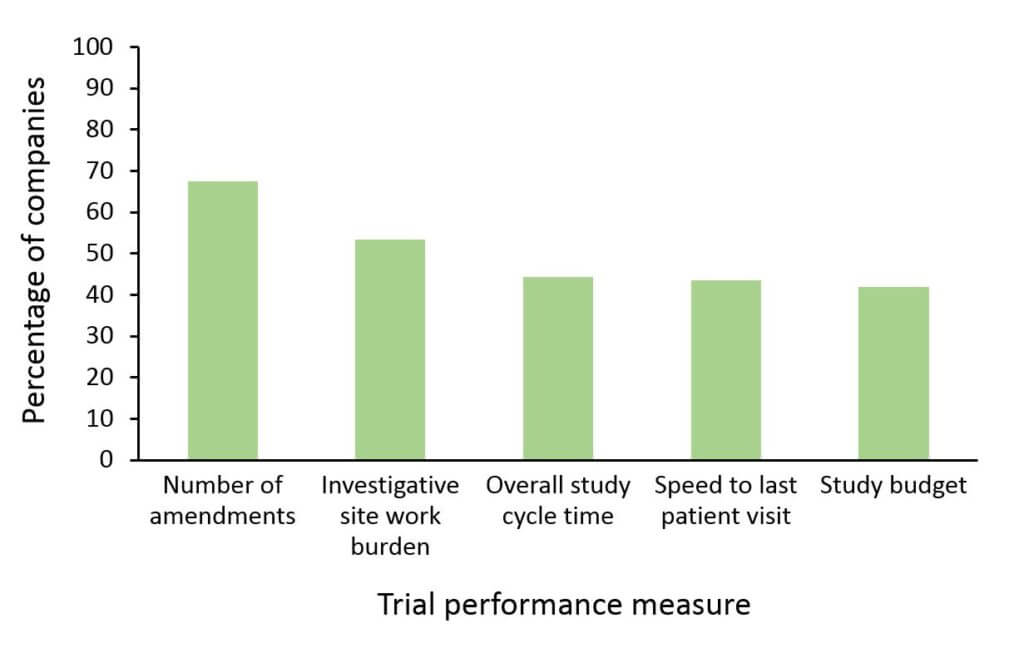
A discussion of social media perceptions with 20 pharmaceutical and biotechnology companies, as detailed in the report, further revealed that companies see potential value in soliciting stakeholder (e.g., investigators, patients) input on study design via social media. However, serious concerns regarding lack of regulatory guidance and potential for bias have kept companies from implementing this type of engagement to date.
Reference
1. Tufts Center for the Study of Drug Development. Protocol design optimization starting to improve study performance. Tufts CSDD Impact Report. 2014;16(5).


