
A collaborative care intervention for injured patients at trauma centers can reduce symptoms of posttraumatic stress disorder (PTSD), according to the results of the Trauma Survivors Outcomes and Support (TSOS) study. The results were published online this week in JAMA Surgery.
The TSOS study, an NIH Collaboratory Demonstration Project, was a stepped-wedge, cluster randomized pragmatic clinical trial testing the delivery of a stepped collaborative care intervention vs usual care for 635 injured patients with PTSD symptoms and comorbid conditions at 25 level I trauma centers in the United States.
Patients in the control group received usual care plus nurse notification about the patient’s high level of distress. Patients in the intervention group received collaborative care consisting of evidence-based medication, cognitive behavioral therapy, and case management. Patients in the intervention group whose PTSD symptoms persisted after initial treatment received stepped-up care, such as medication adjustments or additional psychotherapeutic elements.
After 6 months, the intervention group experienced a significant reduction in PTSD symptoms as compared with the control group. The treatment effect was greater for patients with higher baseline PTSD risk.
“Our study shows that a brief, approximately 2-hour collaborative care intervention delivered by frontline trauma center providers can significantly reduce PTSD symptoms in diverse injury survivors, including survivors of firearm injuries,” said Dr. Doug Zatzick of the University of Washington School of Medicine, the TSOS study’s principal investigator. “Collaborative care may therefore be an optimal treatment approach for trauma-exposed patients treated in acute care medical settings,” he said.
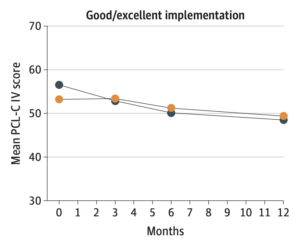
The study also included an implementation process assessment to allow the investigators to examine how the quality of each site’s implementation of the protocol affected study outcomes. The effect of the intervention on PTSD symptoms was greater at trauma centers with good or excellent protocol implementation.
“The upfront integration of an implementation process assessment into the TSOS pragmatic trial design allowed us to identify sites with good/excellent versus fair/poor protocol implementation, a key characteristic in discriminating patient-level PTSD treatment effects,” Zatzick said.
Overall, reduction in PTSD symptoms did not differ significantly between the study arms at 3 and 12 months, and there was no effect on secondary outcomes. However, the subgroup of patients who had firearm injuries and who were treated at sites with good or excellent implementation had among the largest 6-month treatment effects and demonstrated significant treatment effects at 12 months.
TSOS was supported within the NIH Collaboratory by a cooperative agreement from the National Institute of Mental Health and by the NIH Common Fund through a cooperative agreement from the Office of Strategic Coordination within the Office of the NIH Director. Learn more about the NIH Collaboratory Demonstration Projects.


