What is a Pragmatic Clinical Trial?
Section 4
Pragmatic Elements: An Introduction to PRECIS-2
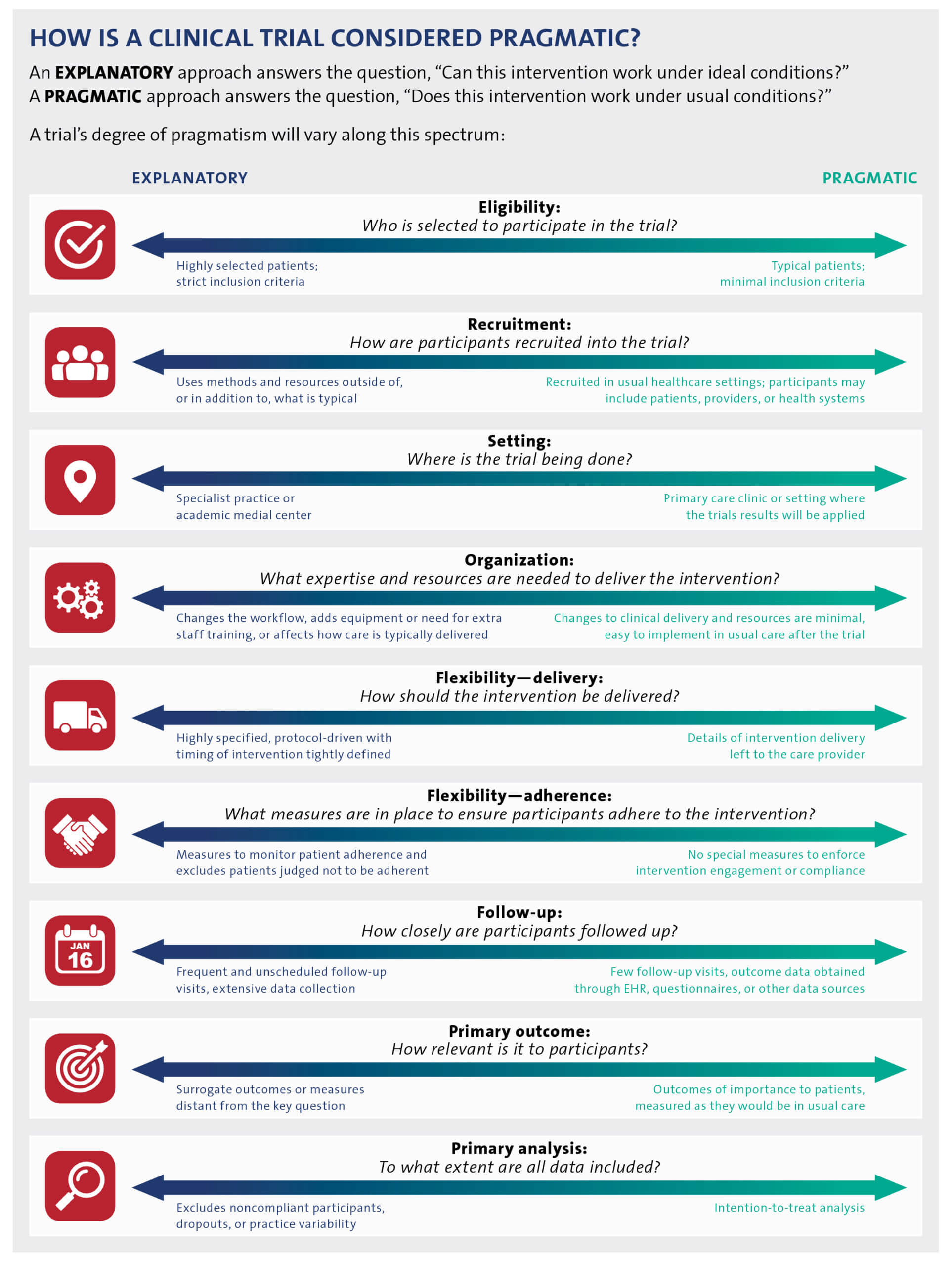
Clinical trials that are considered “pragmatic” are designed to study a health intervention in a real-world setting that is similar or identical to the one in which the intervention will be implemented. PCTs thus stand in contrast to explanatory, or traditional, trials, which are usually designed to demonstrate the safety and efficacy of an intervention under highly controlled conditions and in carefully selected groups of participants.
Most clinical trials are situated somewhere along the spectrum between pragmatic and explanatory. The Pragmatic–Explanatory Continuum Indicator Summary (PRECIS; revised in 2015 as PRECIS-2) offers a framework to guide study teams to prospectively consider the pragmatic or explanatory nature of their trial across 9 domains (Loudon et al. 2015).
Watch the video module: Designing Pragmatic Trials That Are Fit for Purpose
Pragmatic–Explanatory Continuum
While ePCTs are designed to answer important, real-world clinical questions, tradeoffs in flexibility, adherence, and generalizability are inevitable. An ePCT may have some elements that are more pragmatic and some that are more explanatory. When planning the trial, it is essential to first ask, “What is the best trial design to answer the question of interest?” There is nothing inherently better or worse about a pragmatic versus traditional design. Rather, investigators should choose the degree of pragmatism for each element of the design (Figure) that will best answer the research question of interest. To evaluate the degree to which a trial is pragmatic, study teams are encouraged to read the detailed explanations of PRECIS-2, along with examples of how to apply the tool (Loudon et al. 2015).
PRECIS-2 Wheel Diagram
In 2016, members of the Health Care Systems Interactions Core and colleagues applied the PRECIS-2 criteria to 5 PCTs supported by the NIH Collaboratory. Each trial was found to be “highly pragmatic” across the PRECIS-2 domains, highlighting the tool’s potential usefulness in guiding decisions about study design but also revealing a number of challenges in applying it and interpreting the results (Johnson et al. 2016). As another example, during the conduct of the PROVEN NIH Collaboratory Trial, members of the study team applied the PRECIS-2 domains in a novel manner to assess how dynamic adaptations shifted implementation to either a more explanatory or a more pragmatic approach (Palmer et al. 2018).
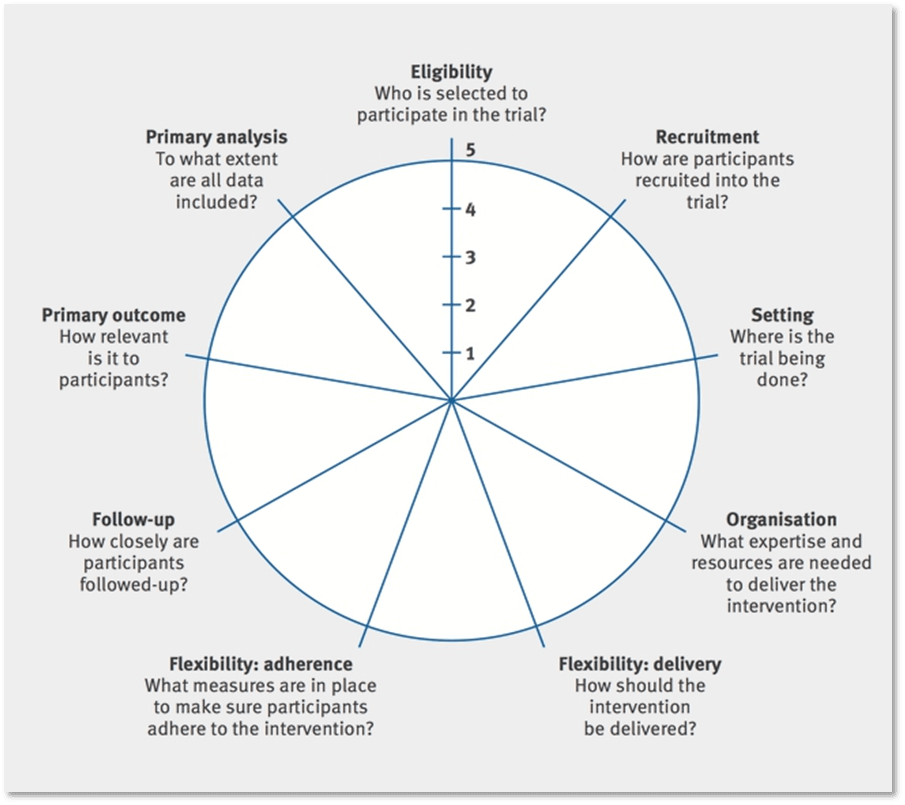
PRECIS-2: Kirsty Loudon et al. BMJ 2015;350:bmj.h2147. Copyright 2015 by British Medical Journal Publishing Group. Used by permission.
- What would a PRECIS-2 wheel diagram look like for the trial you are developing?
The following Table illustrates key characteristics and examples of interventions that are more pragmatic than explanatory.
Characteristics and Examples of PCT Elements
| Trial element | Pragmatic characteristic | Example |
| Research question | Tests whether the intervention is effective in routine clinical practice |
|
| Setting | Embedded in the routine care setting such as primary care, community clinics, hospital units, or health systems |
|
| Participants | Eligible population requires little selection beyond the clinical indication of interest |
|
| Intervention and comparator | Compares two or more real-world treatments using flexible protocols |
|
| Outcomes | Endpoints are clinically relevant to participants, funders, communities, and healthcare providers |
|
| Clinical importance | Purposely designed for making healthcare decisions in settings in which the intervention will be implemented |
|
Elements Adapted from Zwarenstein et al. 2008.
SECTIONS
Resources
View a brief video introduction to PRECIS-2
Visit the PRECIS-2 website for more information on how to understand and use the tool
Members of the NIH Collaboratory’s Health Care Systems Interactions Core applied retrospective PRECIS-2 ratings to 5 NIH Collaboratory Trials, described in Johnson et al. Trials 2016
REFERENCES
Johnson KE, Neta G, Dember LM, et al. 2016. Use of PRECIS ratings in the National Institutes of Health (NIH) Health Care Systems Research Collaboratory. Trials. 17:32. doi:10.1186/s13063-016-1158-y. PMID:26772801.
Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. 2015. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 350:h2147. doi:10.1136/bmj.h2147. PMID: 25956159.
Palmer JA, Mor V, Volandes AE, et al. 2018. A dynamic application of PRECIS-2 to evaluate implementation in a pragmatic, cluster randomized clinical trial in two nursing home systems. Trials. 19:453. doi:10.1186/s13063-018-2817-y. PMID: 30134976.
Zwarenstein M, Treweek S, Gagnier JJ, et al. 2008. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ. 337. doi:10.1136/bmj.a2390. PMID:19001484.


